New cases of coronavirus infections in Germany have soared to 6,638 in the past 24 hours, official data showed Thursday, reaching a daily level not seen since the start of the pandemic.The alarming jump in numbers came just hours after Chancellor Angela Merkel met with the leaders of Germany’s 16 federal states to agree tougher restrictions designed to slow the spread of the contagion. The highest number of new cases previously recorded in one day was 6,294, on March 28, according to figures from the Robert Koch Institute disease control centre. Chancellor Angela Merkel on Wednesday night announced new limits on people gathering at events as well as mandatory mask wearing in crowded places. Under the new measures, if an area records more than 35 new infections per 100,000 people over seven days, masks will become mandatory in all places where people have close contact. “We can see that … infection rates are rising and that we have a very high infection rate in some regions,” Merkel said. “We must therefore prevent an uncontrolled or exponential increase.” The number of people allowed to gather will also be limited to 25 in public and 15 in private spaces. Once a threshold of 50 new infections per 100,000 is exceeded, even tougher restrictions will apply. These include limiting private gatherings to 10 people or two households, and the closure of restaurants after 11:00 pm.Even more curbs could be imposed if the upwards trajectory of new infections is maintained, Merkel warned. “We will see if what we’ve done today is enough,” she said after Wednesday’s decisions.
Melania Trump says their son, Barron Trump, tested positive for COVID-19
https://youtu.be/UtKy1pkAjkw
Melania Trump said Wednesday that after an initial negative test, 14-year-old Barron Trump tested positive for COVID-19. “It was two weeks ago when I received the diagnosis that so many Americans across our country and the world had already received—I tested positive for COVID-19,” the first lady said in a statement. “To make matters worse, my husband, and our nation’s Commander-in-Chief, received the same news.” She continued: “Naturally my mind went immediately to our son. To our great relief he tested negative, but again, as so many parents have thought over the past several months, I couldn’t help but think “what about tomorrow or the next day?” My fear came true when he was tested again and it came up positive. Luckily he is a strong teenager and exhibited no symptoms. In one way I was glad the three of us went through this at the same time so we could take care of one another and spend time together. He has since tested negative.” Stephanie Grisham, the first lady’s chief of staff and spokeswoman, told USA TODAY on Oct. 2 that Barron “has tested negative, and all precautions are being taken to ensure he’s kept safe and healthy.”
Radio Free Wall Street
Germ Warfare Attack
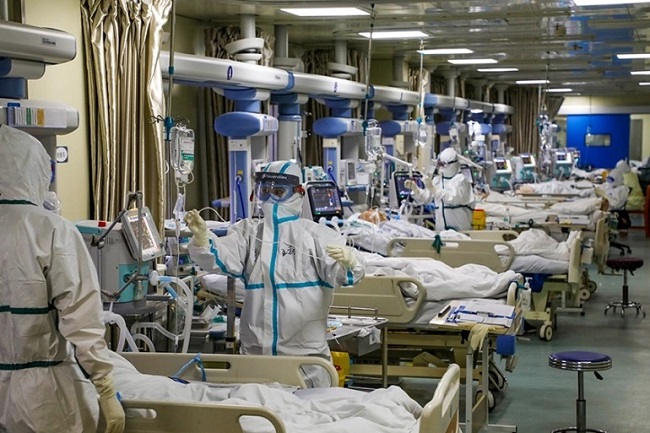
We are under a germ warfare attack by the Chinese. I don’t speak these words lightly and I have triple checked my facts. The Chinese have eradicated the Coronavirus from their country. They are the only society that has returned to 100% normal. I’m going to present to you the challenge that you face and then I’m going to present solutions.
Trump again attacks Fauci’s guidance as coronavirus infections tick upward
WASHINGTON – President Donald Trump’s long-fraught relationship with Anthony S. Fauci, the nation’s top infectious-disease specialist, ruptured again this week in an ugly public dispute just as U.S. coronavirus cases have ticked past 50,000 per day and with three weeks left in a presidential campaign dominated by the government’s response to the pandemic. Trump on Tuesday responded to Fauci’s warnings that the president’s decision to resume campaign rallies this week was “very troublesome” by mocking him in a tweet that unfavorably compared his medical guidance to his errant ceremonial first pitch at a Washington Nationals’ game in July. “Actually, Tony’s pitching arm is far more accurate than his prognostications,” Trump wrote, erroneously suggesting that Fauci’s advice in the early days of the pandemic that the public need not wear face masks meant that the doctor was playing down the virus.
The president’s nasty personal attack offered more evidence of the large gulf between the government’s top two public messengers on the pandemic, who have consistently contradicted one another. Trump has repeatedly undercut Fauci’s advice and sought to minimize the threat of a virus that has killed at least 215,000 Americans, while the doctor has tacitly criticized the president for failing to follow basic safeguards such as wearing a mask and social distancing.
Trump has not met individually with Fauci in more than a month, White House aides said, keeping tabs mostly by watching his appearances on cable news shows.
The latest tensions, though, illustrate a conundrum for Trump, who has sought to win public confidence with a relatively quick rebound from his own coronavirus diagnosis that forced him into a three-night stay at Walter Reed National Medical Center this month.
Though he has continued to denigrate Fauci, his campaign offered an acknowledgment of the public’s high regard for the director of the National Institute of Allergy and Infectious Diseases by including an out-of-context clip of him in a recent advertisement – a move that angered Fauci.
The ad, which tout the president as “sparing no expense” to protect seniors from the virus, includes a brief video of Fauci saying, “I can’t imagine that anybody could be doing more” – a comment he made during a Fox News interview in March. The full interview makes clear, however, that Fauci was speaking about the White House’s coronavirus task force, of which he is a member, and not about the president.
Fauci, 79, said in a statement Sunday that he had never endorsed a political candidate in his nearly five decades of public service. In an interview with the Daily Beast on Monday, Fauci demanded the Trump campaign remove the clip. “By doing this against my will they are, in effect, harassing me,” he said.
Fauci, who has said he is not considering resigning, declined to comment for this story. Continue reading “Trump again attacks Fauci’s guidance as coronavirus infections tick upward”
U.S. pauses Eli Lilly’s trial of a coronavirus antibody treatment over safety concerns
Eli Lilly’s late-stage trial of its leading monoclonal antibody treatment for the coronavirus has been paused by U.S. health regulators over potential safety concerns, the company confirmed to CNBC on Tuesday. “Safety is of the utmost importance to Lilly. We are aware that, out of an abundance of caution, the ACTIV-3 independent data safety monitoring board (DSMB) has recommended a pause in enrollment,” spokeswoman Molly McCully told CNBC. “Lilly is supportive of the decision by the independent DSMB to cautiously ensure the safety of the patients participating in this study.” The news comes less than 24 hours after Johnson & Johnson confirmed that its late-stage coronavirus vaccine trial was paused after a participant reported an “adverse event” the day before. Dr. Mathai Mammen, global head of the Janssen research and development arm at J&J, told investors on a conference call Tuesday that the company still has “very little information” on the reason for the holdup, including if the patient received the vaccine or the placebo. Preliminary information has been sent to the data safety monitoring board for review, he added. Medical experts note that pauses in large clinical trials are not uncommon. They added it’s possible the bad reaction could be the result of an unrelated illness, and not the drug itself. The review from the data and safety monitoring board will help determine that. The ACTIV-3 trial is designed to test a monoclonal antibody developed by Eli Lilly in combination with Gilead Sciences’ remdesivir, an antiviral with emergency use authorization for the virus. It’s one of several ongoing trials that are part of the National Institute of Health’s “Activ” program, which is designed to accelerate the development of Covid-19 vaccines and treatments. It is also backed by Operation Warp Speed, the Trump administration’s effort to manufacturer and distribute vaccines to fight Covid-19. Eli Lilly’s drug is part of a class of treatments known as monoclonal antibodies, which are made to act as immune cells that scientists hope can fight the virus. The treatment was developed using a blood sample from one of the first U.S. patients who recovered from Covid-19. AstraZeneca and Regeneron, among other companies, are also working on so-called antibody treatments. Monoclonal antibody treatments hit the headlines this month after news broke that President Donald Trump received an antibody cocktail from Regeneron. As Trump’s health improved, he touted it as a “cure.” But Regeneron’s CEO, Leonard Schleifer, has stressed that more testing is required. Trump has previously touted Eli Lilly’s treatment and others. Earlier this month, when he was sick with Covid-19, he said, “We have these drugs, Eli Lilly and the others that are so good.” “They are in my opinion, remember this, they’re going to say that they’re therapeutic. And I guess they are therapeutic. Some people don’t know how to define therapeutic. I view it different. It’s a cure,” Trump said in a video posted Oct. 7 on Twitter. “For me, I walked in. I didn’t feel good. A short 24 hours later, I was feeling great. I wanted to get out of the hospital. And that’s what I want for everybody. I want everybody to be given the same treatment as your president because I feel great.” No details regarding Eli Lilly’s safety concern are yet known. “When scientists test promising treatments, sometimes unexpected side effects occur,” said Jeremy Faust, a health policy expert and emergency medicine doctor at Brigham and Women’s Health in Boston. Faust was part of the group of scientists that first reported the news via the research site Brief19. “When only a small number of patients have received a compound, it’s hard to tell what’s a real problem and what is noise,” he told CNBC. “That’s why patience and prudence are always warranted before doling out experimental treatments.”
Dr. Fauci: The Trump Campaign Is ‘In Effect, Harassing Me’
Dr, Anthony Fauci told the Daily Beast that it would be “outrageous” and “terrible” if he was featured in another Trump campaign commercial and it could “come back to backfire” on Team Trump. Explained Fauci: “By doing this against my will, they are in effect harassing me. Since campaign ads are about getting votes, their harassment of me might have the opposite effect of turning some voters off.”
Senate to vote on PPP funding next week – McConnell
https://youtu.be/QeLGiLKKfk0?t=29
Senate Majority Leader Mitch McConnell (R-Ky.) issued a statement on Tuesday saying that the Senate’s “first order of business” when it returns on Oct. 19 will be to vote on “targeted relief for American workers,” including new funding for the small business Paycheck Protection Program (PPP).
Why it matters: House Democrats, Senate Republicans and the Trump administration are still very far apart on key elements of a relief deal, and any push for smaller, more targeted legislation is more of a political maneuver than any thing else.
- The Senate has largely been left out of the negotiating process between House Speaker Pelosi and the White House, and any deal that results from those conversations will likely not be supported by Senate Republicans.
- This is McConnell’s way of showing Senate Republicans are still dedicated to some form of coronavirus stimulus, and could serve as a backstop in the event that a new bill is passed in the House that Republicans in the Senate deem unworkable.
Driving the news: McConnell’s statement comes one month after Senate Democrats filibustered a Republican “skinny bill” that focused on school aid, unemployment benefits and assistance for small businesses. Democrats called the package “piecemeal” and said it was “laden with poison pills Republicans know Democrats would never support.”
What they’re saying: “The PPP is a popular program that has saved tens of millions of American jobs. It is so bipartisan that its first round was replenished and extended several times by unanimous consent in both the Senate and the House,” McConnell said in a statement.
- “Democrats have spent months blocking policies they do not even oppose. They say anything short of their multi-trillion-dollar wish list, jammed with non-COVID-related demands, is “piecemeal” and not worth doing.”
- “Speaker Pelosi frequently says she feels “nothing” is better than “something.” And she has worked hard to ensure that nothing is what American families get.”
Meanwhile: President Trump, who called off stimulus negotiations with Democrats last week, tweeted on Tuesday morning, “STIMULUS! Go big or go home!!!” It’s unlikely that a package the size that Trump and Democrats are negotiating will win the support of Senate Republicans.
You really DO NOT want to take a monkey vaccine

THE VIRUS AND THE VACCINE
Continue reading “You really DO NOT want to take a monkey vaccine”
J & J Covid-19 vaccine study paused due to unexplained illness in participant
The study of Johnson & Johnson’s Covid-19 vaccine has been paused due to an unexplained illness in a study participant. A document sent to outside researchers running the 60,000-patient clinical trial states that a “pausing rule” has been met, that the online system used to enroll patients in the study has been closed, and that the data and safety monitoring board — an independent committee that watches over the safety of patients in the clinical trial — would be convened. The document was obtained by STAT. Contacted by STAT, J&J confirmed the study pause, saying it was due to “an unexplained illness in a study participant.” The company declined to provide further details. “We must respect this participant’s privacy. We’re also learning more about this participant’s illness, and it’s important to have all the facts before we share additional information,” the company said in a statement. J&J emphasized that so-called adverse events — illnesses, accidents, and other bad medical outcomes — are an expected part of a clinical study, and also emphasized the difference between a study pause and a clinical hold, which is a formal regulatory action that can last much longer. The vaccine study is not currently under a clinical hold. J&J said that while it normally communicates clinical holds to the public, it does not usually inform the public of study pauses. The data and safety monitoring board, or DSMB, convened late Monday to review the case. J&J said that in cases like this “it is not always immediately apparent” whether the participant who experienced an adverse event received a study treatment or a placebo. Though clinical trial pauses are not uncommon — and in some cases last only a few days — they are generating outsized attention in the race to test vaccines against SARS-CoV-2, the virus that causes Covid-19. Given the size of Johnson & Johnson’s trial, it’s not surprising that study pauses could occur, and another could happen if this one resolves, a source familiar with the study said. “If we do a study of 60,000 people, that is a small village,” the source said. “In a small village there are a lot of medical events that happen.” On Sept. 8, a large study of another Covid-19 vaccine being developed by AstraZeneca and Oxford University was put on hold because of a suspected adverse reaction in a patient in the United Kingdom. It’s believed that the patient had transverse myelitis, a spinal cord problem. Studies of the vaccine resumed roughly a week after it was paused in the United Kingdom, and have since been restarted in other countries as well. It remains on hold, however, in the United States. Johnson and Johnson began enrolling volunteers in its Phase 3 study on Sept. 23. Researchers planned to enroll 60,000 participants in the United States and other countries.
Radio Free Wall Street
You’ll Need Your Tinfoil Hat For This One

It’s time I tell you the rest of my Wuhan story. There is a reason in my dream that the Chinese were taking me off to my death. And it’s sad for me to report that the other 3 people that were at the table with me in that dream are either dead or missing.