You should get a COVID-19 vaccine even if you already had COVID-19. No currently available test can reliably determine if you are protected after being infected with the virus that causes COVID-19.
Getting a COVID-19 vaccine after you recover from infection with the virus that causes COVID-19 provides added protection to your immune system. People who already had COVID-19 and do not get vaccinated after their recovery are more likely to get COVID-19 again than those who get vaccinated after their recovery.
If you currently have COVID-19, you should wait to get your vaccine until your symptoms are gone (if you had symptoms) and you are done with your isolation period. If you are not vaccinated and were exposed to someone with COVID-19, you should wait until your quarantine is over to avoid getting others sick while you get your vaccine.
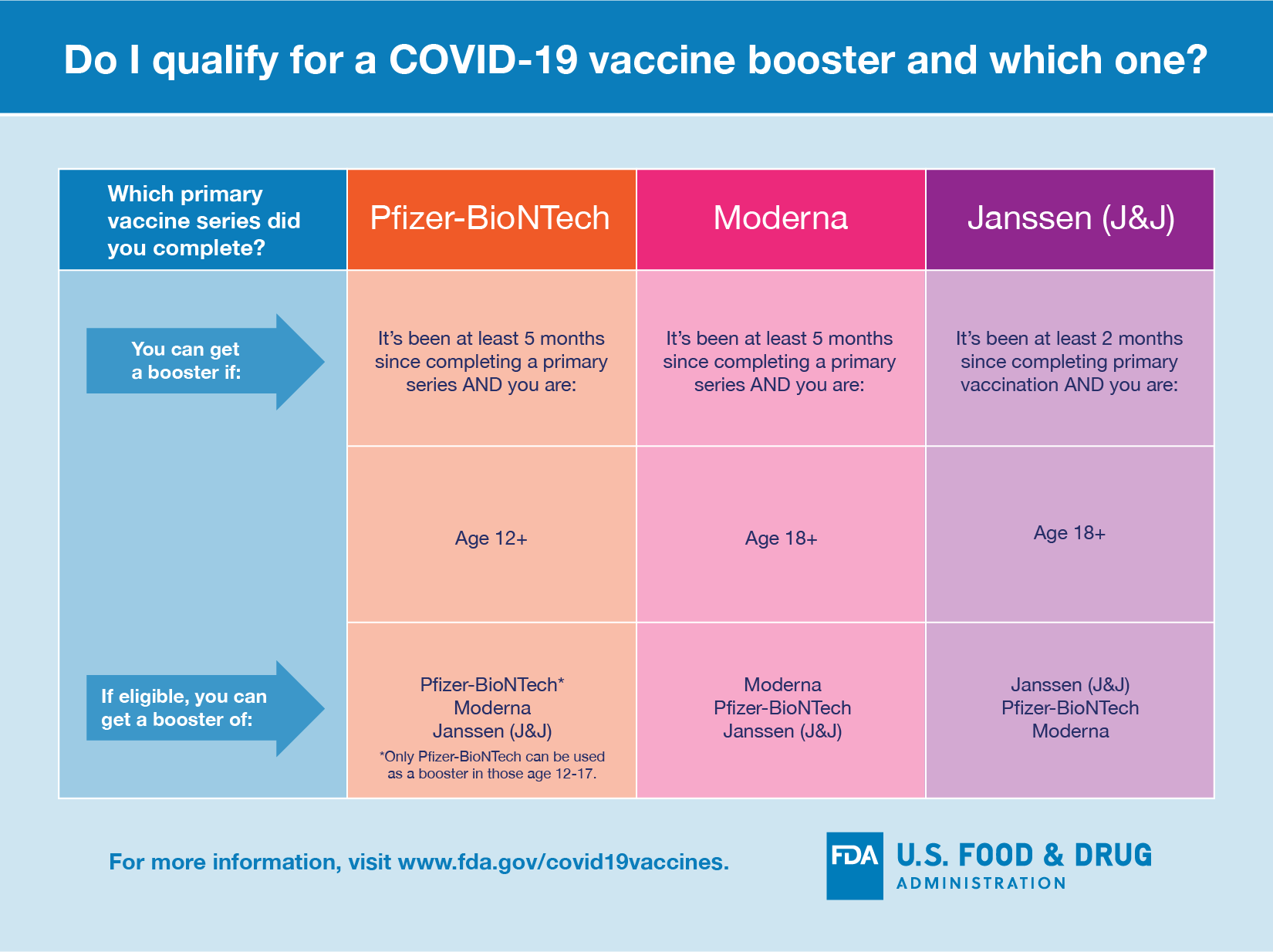
Note: CDC recommends that all people with HIV get a COVID-19 vaccination, as well as a booster shot when they are eligible. People who are moderately or severely immunocompromised—including those with advanced or untreated HIV—have specific COVID-19 vaccine recommendations, which include an additional primary dose, as well as a booster shot for those eligible. NN: the first line of defense is getting vaccinated unfortunately every 5 or 6 months… It’s your body and your choice…. I believe in the war on Covid you should use ALL the weapons at your disposal. I believe setting the captives free, will be proven to be a colossal mistake by September.