Starbucks plans to reopen 85 percent of its locations under modified operations and hours across the US by the end of the week, the coffee giant announced. The company’s mobile app — already used by about 20 million customers — will be optimized for voice ordering through Siri, curbside pickup, entryway handoff, and improved drive-thru experiences, according to a letter CEO and president Kevin Johnson wrote to customers Monday. “We are putting immense emphasis on the safest and most convenient way for customers to order their favorites from Starbucks,” Johnson said. The company will “shift toward more cashless experiences, knowing that the handling of cash creates consumer concerns about the spread of viruses,” according to Johnson. “We predict the mobile app will become the dominant form of payment,” he said. A Starbucks spokesman said the company does not yet have specific details about openings in the Big Apple. Even before the pandemic, more than 80 percent of orders at U.S. Starbucks were placed “on the go” through a drive-through or mobile app, according to Johnson. The Seattle-based chain temporarily shuttered about 8,000 of its company-owned stores across the country in late March — and is one of the first national chains to share its reopening plans, The Washington Post reported. By early June, more than 90 percent of Starbucks locations are expected to be back in business, Johnson said.
Fever-reading drones just first of a wave of privacy challenges, civil liberties advocates say
MIAMI — Last month, police departments in Daytona Beach, Fla., and Connecticut unveiled what was initially touted as a potential new tool against a pandemic: drones capable of taking a person’s temperature from 300 feet in the air. Both agencies quickly backtracked on using the machines to track the novel coronavirus after backlash from civil liberty groups warning about the implications of a “Big Doctor” in the sky singling out people simply for running a fever, when it might be nothing more than a more common and less deadly flu. They raised other concerns as well: Are cops supposed to be monitoring health information that is private under federal law? Are drone readings, even with sophisticated infrared sensors, a trustworthy way to protect public health without violating individual rights? “It collects data and information on everybody without guaranteeing it’s accurate,” said Kara Gross, legislative director of the American Civil Liberties Union of Florida. “Not only that, the other people around the person may have COVID. So the information could be bad and inaccurate.”
The drones are just one example of what some civil rights advocates fear could be a looming wave of intrusive technology and constitutionally questionable measures pushed by governments — from local to state to federal — under the mission of protecting a fearful community. Continue reading “Fever-reading drones just first of a wave of privacy challenges, civil liberties advocates say”
Italy Reports Lowest Toll Since First Day of Coronavirus Lockdown But Confusion Abounds
Cellphone monitoring is spreading with the coronavirus. So is an uneasy tolerance of surveillance.
US FDA approves Roche’s coronavirus antibody test
Roche Holding AG received emergency-use authorization from the U.S. Food and Drug Administration for its new Covid-19 antibody test, according to a company statement. Governments are trying to learn how many people have been exposed to Covid-19 as they reopen economies and end social-distancing measures. The restrictions have resulted in millions of lost jobs, closed schools and businesses and sent the financial markets through the most turbulent period since the 2008 financial crisis. With no vaccine available yet for the novel coronavirus, more than 100 different programs have been launched to develop and test treatments. These include everything from antiviral drugs and antibody-containing plasma from recovered patients, to traditional Chinese herbal medicine. Gilead Sciences Inc.’s antiviral drug remdesivir was cleared by U.S. regulators on May 1 for emergency use in Covid-19 patients, becoming the first medication backed by early clinical data to be made available to fight the disease. The Elecsys Anti-SARS-CoV-2 test is designed to determine if a patient has been exposed to the coronavirus and has developed antibodies against it, Roche said on Sunday.
Government researchers changed metric to measure coronavirus drug remdesivir during clinical trial
Government clinical trial investigators changed the primary metric for measuring the success of Gilead’s experimental drug remdesivir as a coronavirus treatment two weeks before Anthony S. Fauci’s announcement that the drug would be the new “standard of care.” Instead of counting how many people taking the drug were kept alive on ventilators or died, among other measures, the National Institute of Allergy and Infectious Diseases said it would judge the drug primarily on a different outcome: how long it took surviving patients to recover. Death and other negative outcomes were moved to secondary measure status: They would still be tracked, but they would no longer be the key measure of remdesivir’s performance. The switch — which specialists said is unusual in major clinical trials but not unheard of — was publicly disclosed on the government’s clinicaltrials.gov website on April 16 but did not receive much attention at the time. The change reflects evolving scientific understanding of the fast-moving nature of the virus and uncertainties around how the lethal effects reveal themselves in patients, said NIAID, Gilead, and outside specialists. But the change also adds weight to the assessment of government and medical researchers that remdesivir is not a knockout drug that will change the trajectory of the coronavirus pandemic. On Friday, as expected, the Food and Drug Administration approved an emergency use authorization for the drug that will allow it to be prescribed for hospitalized patients infected with the coronavirus. The newly adopted criteria were a central feature of this week’s declaration by Fauci, NIAID’s director, that remdesivir reduced the time to recovery for surviving patients from 15 days to 11 days, a 31 percent improvement. “The data shows that remdesivir has a clear-cut, significant, positive effect in diminishing the time to recovery,” Fauci said as he sat in an Oval Office meeting with President Trump and other members of the president’s coronavirus task force. “It’s highly significant.” The difference in death rate, one of the original primary measures, was not statistically significant, Fauci said, showing only a marginal reduction from 11 percent in patients given a placebo to 8 percent in patients given remdesivir. Full release of the trial results would be made soon, Fauci said. Some medical research specialists questioned the change in the primary outcome measure of the trial, which had 1,063 patients. “I think that they thought they weren’t going to win, and they wanted to change it to something they could win on,” said Steven Nissen, a Cleveland Clinic cardiologist and expert clinical investigator who has led numerous drug trials. “I prefer the original outcome. It’s harder. It’s a more meaningful endpoint. “Getting out of the hospital early is useful,” he said, “but it’s not a game-changer.”
Leaked dossier details China hiding virus info
Bombshell ‘Five Eyes’ Western intelligence dossier claims China lied about human-to-human transmission, ‘disappeared’ whistle-blowers and refused to help other countries prepare a vaccine for coronavirus
Chinas ‘risky’ research on bat-related diseases stretching back years. It describes how Beijing was outwardly downplaying the outbreak on the world stage while secretly scrambling to bury all traces of the disease. This involved ‘destroying’ laboratory samples, bleaching wet market stalls, censoring the growing evidence of ‘silent carriers’ of the virus and stonewalling sample requests from other countries. The secrecy has fanned a clamour in Five Eyes nations for Western governments to come down hard on Beijing when the pandemic eventually passes. Tory MP Bob Seely told MailOnline that ‘at the end of this when the dust settles it is also clear that there has to be a re-evaluation by the West of its relationship with China’. In a damning portrayal of a mass cover-up, the bombshell report reveals:
- Chinese researchers of bat-related viruses studied a sample which had a 96 per cent genetic match to Covid-19 as early as 2013;
- Their ‘risky’ research found in 2015 that the disease was transmissible from bats to humans;
- Information on asymptomatic carriers of the disease was ‘kept silent’ by the Chinese state;
- Beijing started censoring search engines in December to stop any internet surfing relating to the virus;
- The World Health Organisation followed China by denying evidence of human-to-human transmission until late January despite concerns raised by neighbouring countries’;
- The Five Eyes countries lashed out at China for criticising other countries’ flight freezes while simultaneously locking down Hubei Province.
Trump: Gilead drug got emergency use green light from FDA
The Food and Drug Administration will authorize Gilead Sciences Inc.’s remdesivir, a decade-old experimental therapy first tested on Ebola disease patients, as a COVID-19 treatment.
Gilead CEO Daniel O’Day told investors during an earnings call that talks with the FDA about an emergency use authorization (EUA) or a formal approval have intensified over the last 48 hours. “There’s a big sense of urgency,” he said, according to a FactSet transcript of the call. “We think the FDA will move quite quickly.” Shares of Gilead GILD, -4.84% were down 6.9% on Friday following at least three downgrades of the stock on concerns about remdesivir’s moneymaking potential. (“We don’t know what a sustainable revenue stream from remdesivir will look like,” SunTrust Robinson Humphrey analysts wrote in a note.) Year-to-date, the company’s stock has gained 20.4%, hitting a low of $62.23 on Jan. 21 before soaring to a $85.97 high for the year on March 19. The drug has been widely considered a front-runner in the rush to find viable treatments for a disease that has sickened more than three million people worldwide and killed at least 220,000, according to data aggregated by Johns Hopkins University. It would be the first new drug to get an EUA, a type of authorization that the FDA is using during the pandemic. The regulator in March authorized chloroquine and hydroxychloroquine to be repurposed for some COVID-19 patients though both drugs have previously been approved to treat other diseases, like malaria. Investors and clinicians have been paying close attention to the snippets of study data about the antiviral drugs, sending the company’s shares up or down depending on the day. Though experts have had mixed responses to the clinical findings so far, it’s clear that demand is high. There are no proven treatments for infections caused by the coronavirus, making it difficult for the nation’s health-care workers to care for the patients who end up in their emergency rooms and intensive care unit beds. There’s also the economic angle. The market largely swings up in response to remdesivir data, driven by the investor stance that a proven treatment, even more so than vaccine, would support the prospect of an economic rebound for the U.S., which is in its worst recession since the Great Depression. Gilead began developing remdesivir in 2009. It was later tested as a treatment for Ebola, severe acute respiratory syndrome (SARS), and Middle East respiratory syndrome (MERS), among other diseases. The company said it began providing doses of remdesivir to the China Centers for Disease Control and Prevention & Prevention in January when the coronavirus outbreak there began to worsen. By February, the investigational therapy had been moved into a number of clinical trials in the U.S. and abroad, including one conducted by the National Institute of Allergy and Infectious Diseases (NIAID). Government officials including President Trump and Dr. Anthony Fauci, the NIAID director, have talked up the results, with Fauci saying remdesivir would become the standard of care in the treatment of COVID-19 patients, other medical experts have been more measured in their responses to the clinical-trial data. “Under the emergency-use authorization, one could charge for the product,” CEO O’Day told investors on the earnings call. “We made a decision, as you know, to donate 1.5 million vials, which is the entirety of our supply through the early summer.” The vials encompass 140,000 10-day treatment courses. O’Day later said during the call that donating the investigational therapy “is the right thing to do at this time.”
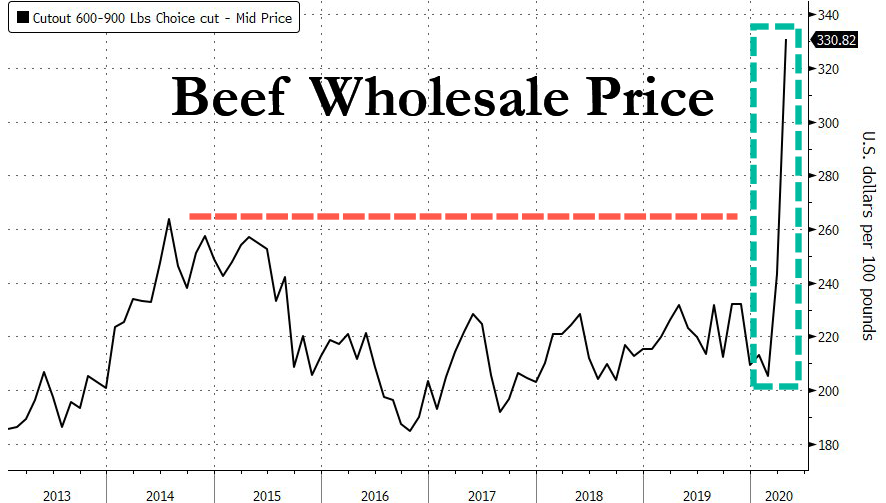
Beef Prices Soar To Record High As Meatpacking Plants Shutter
Wholesale American beef prices jumped 6% to a record high of $330.82 per 100 pounds, a 62% increase from the lows in February, according to Bloomberg, citing new USDA data.
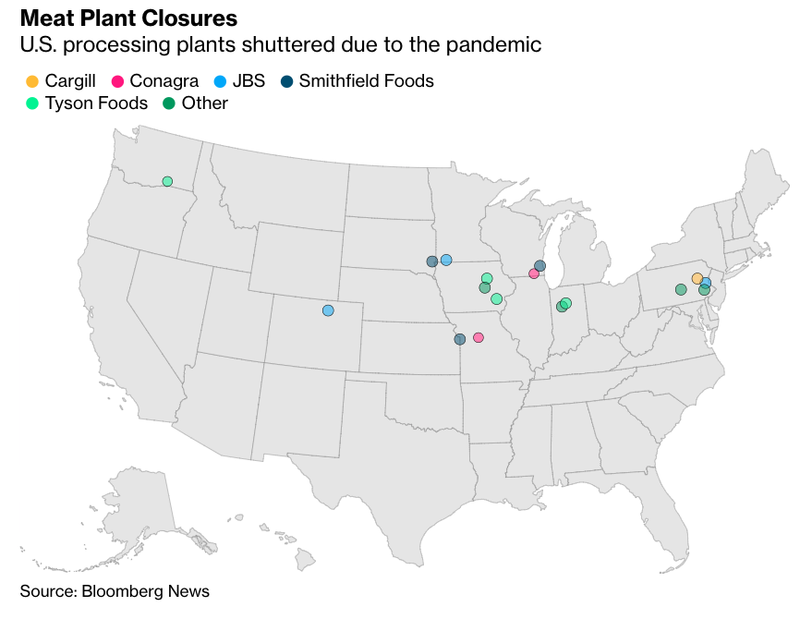
The surge in beef prices comes at a time when the nation’s food supply chain network has been severely damaged by meatpacking plants going offline due to virus-related shutdowns and worker shortage. Bloomberg highlights the latest plant closures in the map below:
Soaring food inflation came one day after President Trump said he would be issuing an executive order to address meat shortages. “Because of the virus, meat slaughtering is 40% below where it needs to be to handle all of the animals coming to market, said Arlan Suderman,” chief commodities economist at INTL FCStone.
“Processing plants were generally in favor of the executive order that would give them liability cover when reopening,” Suderman said. “Yet, the order still does not solve the problem of employee absenteeism.” At least 20 workers in meat and food processing have died and 5,000 have tested positive or forced to self-quarantine due to coronavirus, according to the United Food and Commercial Workers International union.
Just days ago, Tyson Foods warned in a full-page ad in the New York Times on Sunday that the “food supply chain is breaking.” And with tens of millions of Americans out of work, a crashed economy that is plunging into depression, and rapid food inflation — this could all suggest that the evolution of the virus crisis is not just an economic crisis but also social instabilities are ahead.
Trump’s ‘Operation Warp Speed’ Aims to Rush Coronavirus Vaccine
The Trump administration is quietly organizing a Manhattan Project-style effort to drastically cut the time needed to develop a coronavirus vaccine, with a goal to have 100 million doses ready by year’s end, according to two people familiar with the matter. Called “Operation Warp Speed,” the program will pull together private pharmaceutical companies, government agencies and the military to try to cut the development time for a vaccine by as much as eight months, one of the people said. As part of the arrangement, taxpayers will shoulder much of the financial risk that vaccine candidates may fail, instead of drug companies. President Donald Trump’s top medical advisers, led by the infectious disease expert Anthony Fauci, have repeatedly said that a coronavirus vaccine won’t be ready for 12 to 18 months at best. Until then, White House guidelines envision some economically damaging social-distancing practices maintained even as the U.S. begins to resume a more normal social and business life. Last month, Trump directed Health and Human Services Secretary Alex Azar to speed development of a vaccine, and administration officials have been meeting on the effort for three to four weeks, one of the people said. A meeting on the project was scheduled at the White House on Wednesday. The people asked not to be identified because the project hasn’t yet been publicly announced. A spokesman for the Department of Health and Human Services, Michael Caputo, said the president refused to accept the timeline for standard vaccine development and encouraged a breakthrough process. Vaccine development is typically slow and high risk. The project’s goal is to cut out the slow part, the people said. Operation Warp Speed will use government resources to quickly test the world’s most promising experimental vaccines in animals, then launch coordinated human clinical trials to winnow down the candidates. The best prospective vaccines would go into wider trials at the same time mass production ramps up. The project will cost billions of dollars, one of the people said. And it will almost certainly result in significant waste by making inoculations at scale before knowing if they’ll be safe and effective — meaning that vaccines that fail will be useless. But it could mean having doses of vaccine available for the American public by the end of this year, instead of by next summer. There are at least 70 different coronavirus vaccines in development by drugmakers and research groups, according to the World Health Organization. But drugmakers have not coordinated their efforts to the extent they could through the Warp Speed project, one of the people said. The group is also discussing the use of what’s known as a master protocol to test the vaccines. Instead of multiple clinical trials run by each drugmaker, competing for patients and resources, the government would organize one large trial to test several vaccines at once and advance the most promising ones. It’s not clear how much of Operation Warp Speed is new and how much will involve ongoing projects, such as investments made by BARDA, the Biomedical Advanced Research and Development Authority.