A spokeswoman for Donald Trump confirmed on Tuesday morning that the US president is taking a malaria drug as a defense against Covid-19, despite his own administration’s warnings that the drug could have dangerous side-effects. The confirmation came hours after the House speaker, Nancy Pelosi, expressed alarm that Trump was taking the drug since he is “morbidly obese”, in her words. At the White House, the press secretary, Kayleigh McEnany, told CBS News Trump was taking hydroxychloroquine. “I can absolutely confirm that,” she said. “The president said himself he’s taking it. That’s a given fact. He said it. The president should be taken at his word.” Trump told reporters at the White House on Monday that for “a couple weeks” he had been taking hydroxychloroquine, which he first touted as a Covid-19 treatment in March, before the Food and Drug Administration (FDA) warned that the drug could cause irregular heartbeats and other cardiac trouble. The FDA has approved the drug for the treatment of malaria, lupus and rheumatoid arthritis. “You’d be surprised at how many people are taking it … The frontline workers, many, many are taking it,” Trump said on Monday. “I happen to be taking it. I happen to be taking it. I’m taking it, hydroxychloroquine. Right now, yeah. A couple weeks ago I started taking it. Because I think it’s good, I heard a lot of good stories … I take a pill every day.” The White House on Monday night issued a statement by Sean P Conley, Trump’s doctor, that did not quite confirm Trump’s claim to be taking hydroxychloroquine, muddling the issue. “The president is in very good health and has remained symptom-free,” the statement said. “After numerous discussions he and I had regarding the evidence for and against the use of hydroxychloroquine, we concluded the potential benefit from treatment outweighed the relevant risks.” The FDA advised in April that hydroxycholoroquine and chloroquine “have not been shown to be safe and effective for treating or preventing Covid-19”.“While clinical trials are ongoing to determine the safety and effectiveness of these drugs for Covid-19, there are known side-effects of these medications that should be considered,” the FDA commissioner, Stephen Hahn, said in a statement then. The FDA has not updated that guidance. Experts warned that Trump’s claim to be taking the drug could lead to a spike in demand, potentially making it harder for vulnerable patients who need the drug to fill prescriptions.
Moderna may begin final stage vaccine trial in July
Moderna Inc. announced on Monday early-stage trials of the coronavirus vaccine produced positive results so far, adding it expects the final stage of the trials could begin in July. Commenting on the vaccine progress, Moderna’s Chief Medical Officer Tal Zaks revealed that “this interim phase 1 data, while early, demonstrate that vaccination with mRNA-1273 elicits an immune response of the magnitude caused by natural infection starting with a dose as low as 25 micrograms.” Moderna tested the vaccine on 45 males and non-pregnant females aged 18–55, who received two doses with the gap of 28 days between two injections. The company’s stocks surged 27.40% to sell for $83.84 per share in the premarket trade shortly before the start of today’s session.
Operation Warp Speed Vaccination Program
I told you their would be a vaccine even if their is no vaccine
Trump announces ‘Operation Warp Speed,’ says U.S. could have coronavirus vaccine by January
Likening it to the national push to build the atomic bomb during World War II, President Trump on Friday announced Operation Warp Speed, a government coordinating effort aimed at securing a coronavirus vaccine by the end of the year.
“Operation Warp Speed, that means big and it means fast,” Trump said from the White House Rose Garden. “A massive scientific and industrial, logistic endeavor unlike anything our country has seen since the Manhattan Project.”
Its objective is to finish developing and then manufacture and distribute a proven coronavirus vaccine as fast as possible. “We’d love to see if we can do it prior to the end of the year,” Trump said. “I think we’re going to have some very good results coming out very quickly.” The presentation was short on details but noted the initiative is evaluating roughly 100 vaccine candidates from all over the world and has identified more than 14 believed to be the most promising. Officials are working to narrow the list still further. “We have some really interesting choices to be made,” Trump said. The government is providing support and resources to safely expedite trials on those vaccine candidates, “moving on at record, record, record speed,” the President said. Trump confirmed the United States will invest in manufacturing all of the top vaccine candidates before they’re approved. Known as “at risk” production, the government will take on the financial costs, legal liability and clinical trial costs, with no guarantee of getting a usable vaccine. “That means they better come up with a good vaccine,” Trump said. Such extraordinary steps will allow the United States to have vaccine ready as soon as a specific candidate is signed off on as safe and effective by regulatory authorities. The military will be used to help distribute doses. ‘We’re getting ready so that when we get the good word that we have the vaccine, we have the formula, we have what we need, we’re ready to go, as opposed to taking years,” Trump said. Trump acknowledged the risk and expense but said, “we’ll be saving years if we do this properly.” The initiative brings together expertise from the National Institutes for Health, the Centers for Disease Control and Prevention, the Food and Drug Administration and other agencies.The partnership also joins the resources of the Department of Health and Human Services together with the Department of Defense. The national project will bring together the best of American industry and innovation, the full resources of the United States government and the excellence and precision of the United States military, Trump said. In order to make a vaccine available to the entire American population by January, the Food and Drug Administration mayissue an emergency use authorization. Such orders allow unapproved medical products to be used during a public health crisis, without the benefit of the validated testing that would normally take place. “We’re working for a fully approved vaccine but we’ll also use … all of our regulatory tools appropriate to bring vaccine available for the entire American population by January,” Secretary of Health and Human Services Alex Azar said at the news conference. “There’s never been an emergency use authorization for a vaccine before because it’s by and large a technology that is given to healthy people,” said Thomas Bollyky, who directs the global health program at the Council on Foreign Relations.
“It’ll go away at some point, it’ll go away,” he said. “Vaccine or no vaccine, we’re back.”
Democrats push new $3T coronavirus relief bill through House
WASHINGTON (AP) — Democrats have powered a massive $3 trillion coronavirus relief bill through the House, an election-year measure designed to brace a U.S. economy in free fall and a health care system struggling to contain a pandemic still pummeling the country. Friday’s 208-199 vote, with all but one Republican opposed, advances what boils down to a campaign-season display of Democratic economic and health-care priorities. It has no chance of becoming law as written, but will likely spark difficult negotiations with the White House and Senate Republicans. Any product would probably be the last major COVID-19 response bill before November’s presidential and congressional elections. The enormous Democratic measure would cost more than the prior four coronavirus bills combined. It would deliver almost $1 trillion for state and local governments, another round of $1,200 direct payments to individuals and help for the unemployed, renters and homeowners, college debt holders and the struggling Postal Service. “Not to act now is not only irresponsible in a humanitarian way, it is irresponsible because it’s only going to cost more,” warned House Speaker Nancy Pelosi, D-Calif. “More in terms of lives, livelihood, cost to the budget, cost to our democracy.”
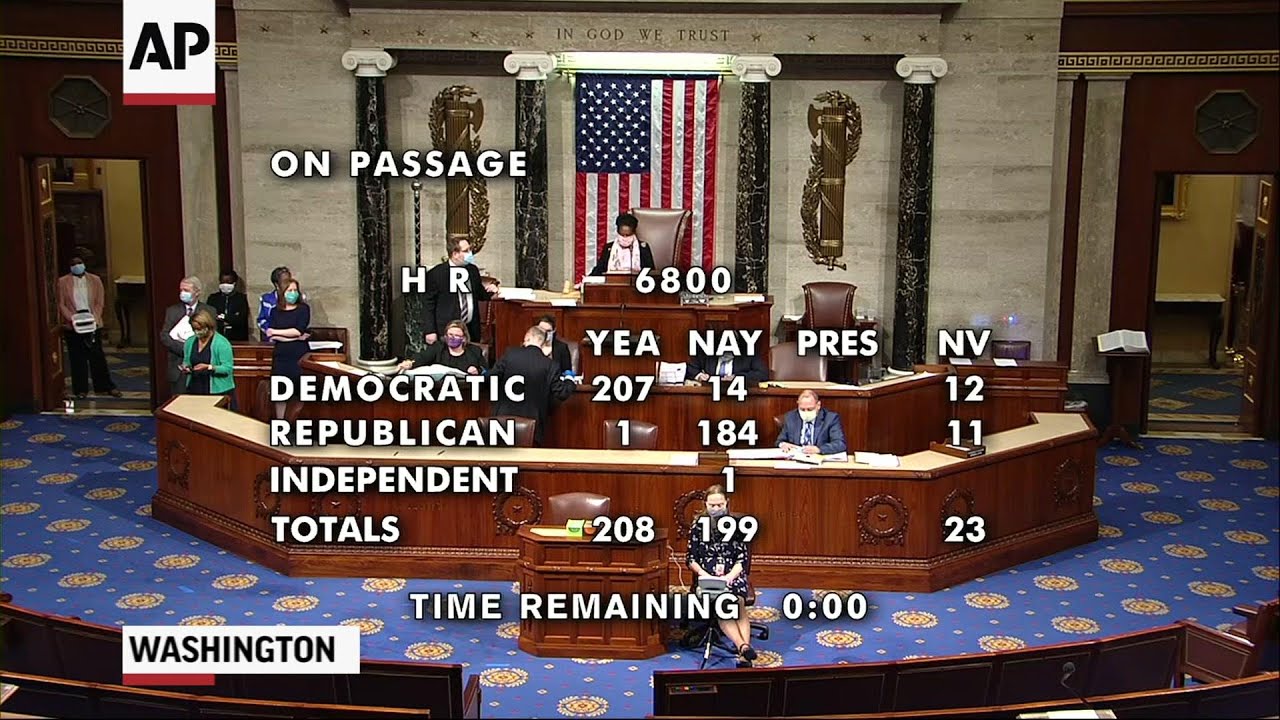
Continue reading “Democrats push new $3T coronavirus relief bill through House”
Most states still fall short of recommended testing levels
WASHINGTON (AP) — As businesses reopened Friday in more of the U.S., an overwhelming majority of states still fall short of the COVID-19 testing levels that public health experts say are necessary to safely ease lockdowns and avoid another deadly wave of outbreaks, according to an Associated Press analysis. Rapid, widespread testing is considered essential to tracking and containing the coronavirus. But 41 of the nation’s 50 states fail to test widely enough to drive their infections below a key benchmark, according to an AP analysis of metrics developed by Harvard’s Global Health Institute. Among the states falling short are Texas and Georgia, which moved aggressively last month to reopen stores, malls, barbershops and other businesses. As health authorities expand testing to more people, the number of positive results should shrink compared with the total number of people tested. The World Health Organization and other health researchers have said a percentage above 10% indicates inadequate testing. South Korea, a country praised for its rapid response, quickly pushed its positive cases to below 3%. Most governors are moving ahead with unlocking their states, even in cases where they are not meeting broad guidelines recommended by the White House. Texas Gov. Greg Abbott has set a goal of 30,000 tests per day as his state launched one of the nation’s most aggressive reopenings on May 1. He never set a firm date on when the state would hit the 30,000 mark, but for most of May, the daily testing numbers have fallen well short of that. Local leaders say tests are still in short supply. El Paso officials have pleaded with the governor to postpone easing up any more business restrictions in light of the COVID-19 cases there surging 60% over the past two weeks. The first stage of reopening in Maryland was scheduled to take effect Friday evening, when some retail stores will be allowed to reopen and a stay-at-home order lifted. But some of the hardest-hit parts of the state, including the suburbs of Washington, D.C., extended restrictions for residents and businesses. Maryland averaged 4,265 tests per day this week, compared with about 4,900 the previous week. Nearly 22 percent of people tested positive in Maryland on average over the last seven days. Maryland Gov. Larry Hogan made headlines last month when the state acquired 500,000 test kits from a South Korean company in a confidential deal, but Maryland has not had all the components needed for testing — like swabs — to meet demand. Hogan said Maryland just received swabs this week from the Federal Emergency Management Agency. “We requested 350,000,” Hogan said Wednesday. “They’ve committed to 225,000, and I think we got 75,000 yesterday with another 125,000 that are supposedly days away, along with the tubes and the stuff that goes with them. So it’s not enough, but it helps us.” Continue reading “Most states still fall short of recommended testing levels”
Cellphone Data Shows 25 Million More Americans Left Home Last Week Compared to Height of Lockdown
is it worth dying for?
It seems Americans didn’t need much encouragement to start leaving their homes again and heading back out into public areas. Cellphone data analyzed by The New York Times reportedly shows that, on average, 25 million more Americans left their homes each day last week compared to the preceding six weeks. The average share of people staying home stood at just over 36 percent, which is said by researchers to be a drop of over 7 percentage points from the average during the peak period for sheltering in place. The surge came as more than half of U.S. states started to reopen their economies or began making plans to do so—even though public-health experts have repeatedly warned that the reopenings will likely cause fresh spikes of infections and deaths. The Times reports the share of people staying home last week dropped in nearly every part of the nation, in some places by nearly 11 percentage points.
Fauci warns: More death, econ damage if US reopens too fast
WASHINGTON (AP) — The U.S. government’s top infectious disease expert issued a blunt warning Tuesday that cities and states could “turn back the clock” and see more COVID-19 deaths and economic damage alike if they lift coronavirus stay-at-home orders too fast — a sharp contrast as President Donald Trump pushes to right a free-falling economy.
“There is a real risk that you will trigger an outbreak that you may not be able to control,” Dr. Anthony Fauci warned a Senate committee and the nation as more than two dozen states have begun to lift their lockdowns as a first step toward economic recovery.
The advice from Fauci and other key government officials — delivered by dramatic, sometimes awkward teleconference — was at odds with a president who urges on protests of state-ordered restraints and insists that “day after day, we’re making tremendous strides.” Trump, whose reelection depends to a substantial degree on the economy, talks up his administration’s record with the virus daily. Underscoring the seriousness of the pandemic that has reached Congress and the White House, Fauci and other experts testified from their homes. Committee Chairman Lamar Alexander chaired the hearing from the study in his cabin in Tennessee, although several committee members attended in person in an eerily empty Capitol Hill chamber, masked and sitting 6 feet apart. The tension in balancing people’s safety from the virus, which is still surprising doctors with the sneaky ways it can kill, against the severe economic fallout is playing out in many other countries, too. Italy partially lifted lockdown restrictions last week only to see a big jump in confirmed COVID-19 infections in its hardest-hit region. And Lebanon relaxed a national lockdown late last month but said Tuesday the restrictions are being reinstated for the rest of the week after a spike in reported infections. Continue reading “Fauci warns: More death, econ damage if US reopens too fast”
White House aides rattled after positive coronavirus tests
WASHINGTON – The White House on Saturday scrambled to deal with the fallout from two aides testing positive for the coronavirus, as officials who were potentially exposed responded differently, with some senior members of the pandemic task force self-quarantining while others planned to continue to go to work. Food and Drug Administration Commissioner Stephen Hahn and Centers for Disease Control and Prevention Director Robert Redfield, both task force members, said they are self-quarantining or teleworking for two weeks after exposure to a coronavirus case at the White House. On Saturday night, a spokeswoman for Anthony Fauci, the government’s top infectious diseases official, acknowledged that working from home sometimes will be among the precautions he is taking. But several administration officials said White House staffers were encouraged to come into the office by their supervisors, and that aides who travel with President Donald Trump and Vice President Mike Pence would not stay out for 14 days, the recommended time frame to quarantine once exposed to the virus. The conflicting ways in which officials and aides are responding after two staff members were diagnosed with the coronavirus this past week – Pence spokeswoman Katie Miller and a military valet to the president – continued to raise questions about how the White House is responding to the challenge of maintaining a safe work environment for Trump, Pence and their staff. “The president’s physician and White House operations continue to work closely to ensure every precaution is taken to keep the president, first family and the entire White House complex safe and healthy at all times,” White House spokesman Judd Deere said. “In addition to social distancing, daily temperature checks and symptom histories, hand sanitizer, and regular deep cleaning of all work spaces, every staff member in proximity to the president and vice president is being tested daily for covid-19 as well as any guests.” But the nervousness and concern among White House staffers became more palpable on Saturday, according to people familiar with the matter who, like others, spoke on the condition of anonymity to discuss the tensions. Now that Redfield and Hahn are staying away, some officials said they don’t know if they should keep going to work at the White House. Staffers who had potentially been in contact with Miller were still getting calls on Saturday from officials trying to gauge their exposure to the virus, according to one person who received a call. All White House staffers received a memo from the White House management office on Friday, which encouraged employees to “practice maximum telework” and to “work remotely if at all possible.” The White House will receive “heightened levels of daily cleaning,” according to the memo. It also told employees they must quarantine for 14 days if they leave the Washington region and must report all of their travel. The memo did not suggest that employees wear masks, as the CDC has suggested for all Americans in public spaces. Masks generally protect other people from the person wearing the face covering, rather than preventing the individual from contracting the virus. “We are exercising daily caution by testing [Executive Office of the President] staff who have high proximity to the president and Vice President for covid-19,” the memo says. “For any presumptive positive covid-19 results, the White House medical Unit conducts immediate contact tracing and notifies any affected individuals.” The FDA said late Friday that Hahn began to self-quarantine for two weeks after being exposed to an individual who tested positive. Continue reading “White House aides rattled after positive coronavirus tests”
Coronavirus contact-tracing: World split between two types of app
Countries around the world are developing Covid-19 smartphone apps to limit the spread of coronavirus and relax lockdown restrictions. It’s hoped the information they gather can be used to alert people whether they pose a risk of spreading the contagion, and need to isolate. But, over recent weeks, a split has emerged between two different types of app – the so-called centralised and decentralised versions. Both types use Bluetooth signals to log when smartphone owners are close to each other – so if someone develops Covid-19 symptoms, an alert can be sent to other users they may have infected. Under the centralised model, the anonymised data gathered is uploaded to a remote server where matches are made with other contacts, should a person start to develop Covid-19 symptoms . This is the method the UK is pursuing. By contrast, the decentralised model gives users more control over their information by keeping it on the phone. It is there that matches are made with people who may have contracted the virus. This is the model promoted by Google, Apple and an international consortium. Backers of the centralised model say it can give the authorities more insight into the spread of the virus and how well the app is performing. Supporters of the decentralised approach say it offers users a higher degree of privacy, protecting them from hackers or the state itself revealing their social contacts. In truth, both are unproven at this stage. South Korea, seen as one of the most successful countries at tackling Covid-19, has done it without a contact-tracing app. It has however used other surveillance methods which would be seen as invasive by many. At the start, the centralised approach was seen pioneering. Singapore’s TraceTogether was widely viewed as the one to emulate. But that changed after it emerged the app was only being used by about 20% of the local population, and there had been a resurgence of Covid-19 cases. Part of the problem is that TraceTogether does not work properly when in the background on iPhones because of the way Apple restricts use of Bluetooth. The firm has promised to waive these curbs, but only if apps fall into line with its decentralised system. Singapore has since signalled it will do so as a result. “We are working with Apple and Google to make the app more effective, especially for iOS users,” a spokesman told the BBC. Australia, another early adopter of the centralised approach, launched its CovidSafe app based on TraceTogether, and faced similar issues as a consequence. It too has said it plans to adopt the Apple-Google framework, citing a “big shift in performance of Bluetooth connectivity”. And on Wednesday, Colombia confirmed it too was considering a switch after having to turn off the contact-tracing feature in its CoronApp. “[We need to] minimise the risk of generating unnecessary alerts,” said presidential advisor Victor Munoz. The developer claims that the combination of the two leads to “very accurate contact tracing results without the need for [the Google-Apple interface”. But this has raised privacy concerns, which may have contributed to a fairly high drop-out rate. The Norwegian Institute of Public Health said that as of 28 April, 1.5 million people had downloaded the app, but only 899,142 were actively using it – representing just 20.5% of over-16s in the test zones. India’s contact-tracing app, Aaroya Setu, takes a similar approach to Norway’s. To tackle adoption, the government has ruled all government and private sector workers must use it. Until Apple and Google release their interface, known as an API, it’s impossible to be sure their system will be any more successful. But the list of nations flocking to it keeps growing. Germany surprised many when it confirmed it had been convinced decentralisation was the way to go – it had previously seemed set to go hand-in-hand with France. “We assume that adapting ProteGo Safe to Google and Apple APIs will be necessary,” developers’ notes read. “We assume that adapting… to Google and Apple APIs will be necessary.” Italy’s Immuni announced it too was backing the US tech giants’ initiative on 29 April, praising its stronger guarantee of anonymity. Other countries set to do likewise include:
“The core reason is that centralised systems ask you to upload the people you have seen, and decentralised systems don’t need that data, so they don’t play well together,” explained Prof Michael Veale of the joint Apple-Google DPT3 group.
The American meat shortage is pushing prices to unprecedented heights
Fresh meat prices were up 8.1% at the end of April, as the coronavirus pandemic causes serious issues in the American supply chain. Pork and beef prices could increase by an unprecedented 20% in the coming months, according to a new report from CoBank. With pork and beef production plunging by 35%, CoBank economist Will Sawyer says shortages and price inflation are “nearly assured.”
Fresh meat prices escalated 8.1% in stores, compared to the same period last year, according to Nielsen data for the week ending April 25. Experts expect prices to skyrocket in the coming weeks, as meat processing plants across the US are forced to close due to the coronavirus pandemic. Pork and beef prices could increase by as much as 20% compared to 2019, according to a new report from CoBank, a cooperative bank that is part of the Farm Credit System. A 20% increase would be an unprecedented price hike, according to Will Sawyer, CoBank’s lead animal protein economist. Pork prices have only experienced inflation of more than 10% twice in the last 20 years; neither time did inflation grow to 20%. Pork and beef production has plunged by roughly 35% compared to this time last year, according to the CoBank report. As a result, Sawyer says, grocery stores running out of products and price inflation are “nearly assured.” Some 115 meat and poultry processing plants have reported COVID-19 cases, according to a report from the Centers for Disease Control and Prevention released last week. There have been 4,913 confirmed COVID-19 cases and 20 deaths among workers, as experts say meat processing plants are becoming the next hotspot for the pandemic. A number of massive meat processing plants have been forced to shut down due to COVID-19 cases or operate at a limited capacity, as concerned employees refuse to come to work and new social distancing policies roll out. In an effort to combat shortages, President Trump recently signed an executive order demanding that meat processing plants stay open. With slaughterhouses shutting down, farmers have been forced to kill pigs and destroy inventory instead of selling at a loss. Hog farmers are expected to euthanize seven million pigs in the second quarter alone.